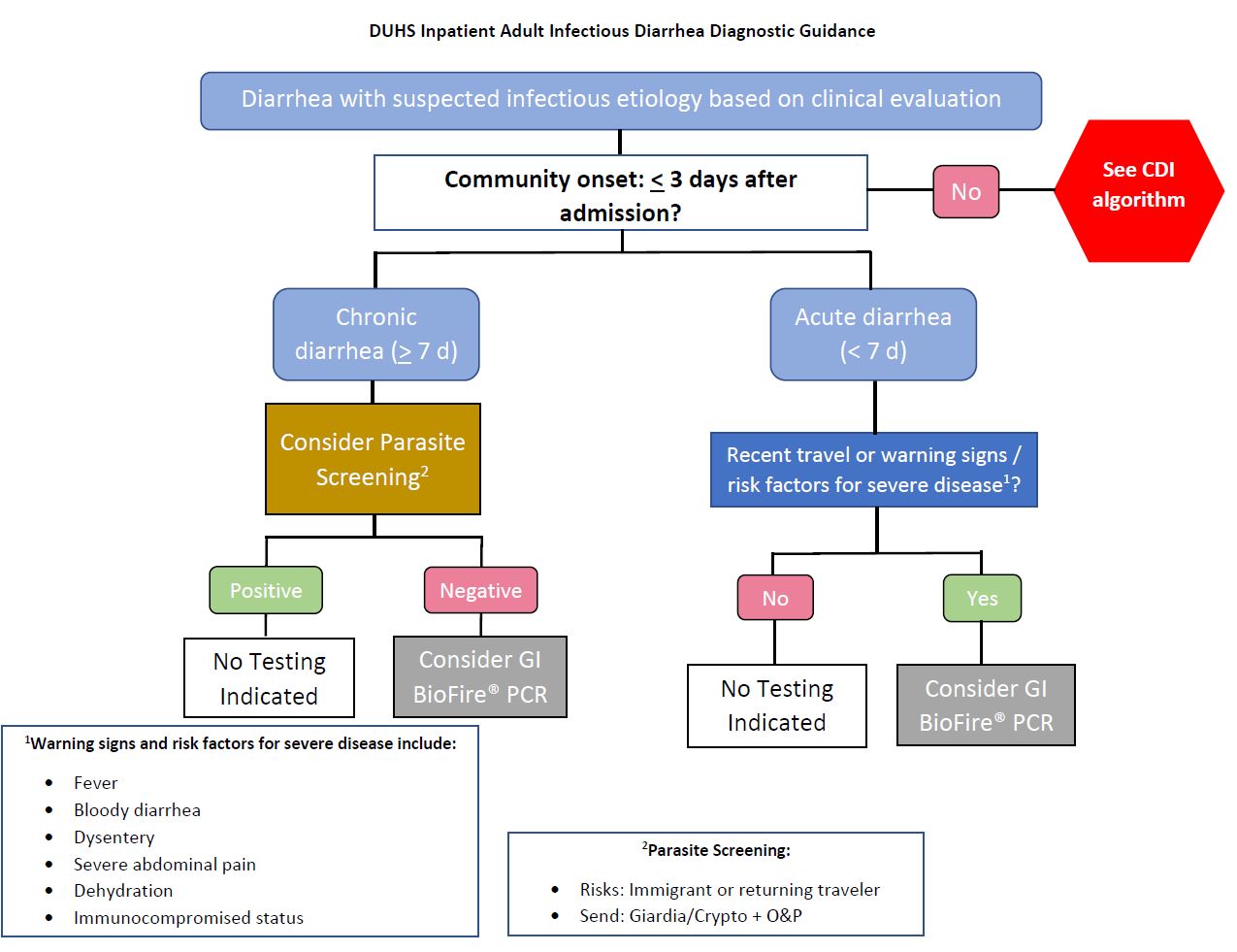
Criteria & Principles
Testing Considerations: Treatment for many GI pathogens does not decrease duration of illness. Only test in certain patient populations to avoid overtreatment and associated harms.
Beginning February 4, 2025, the Duke Clinical Microbiology Laboratory discontinued stool culture and antigen testing and transitioned to a multiplex molecular test for patients with community-onset gastroenteritis with signs of or at risk for severe disease and who are ≤ 3 days from hospital admission. See attached announcement for specifics.
BioFire GI Panel is restricted to the following patient populations:
- Inpatient ≤ 3 days from admission with moderate to severe diarrhea with suspected infectious cause
- Immunocompromised/Transplant patient
For patients with concern for C. difficile infection (CDI), please see the CDI testing algorithm and the separate page for CDI management.
Treatment
Severity
All Severity
Treatment Considerations: Treatment for many GI pathogens does not decrease duration of illness. Recommendations in the table below and in the attached Infectious Diarrhea Management guideline are provided with a goal of minimizing unintended consequences to the patient. Patient-specific factors (e.g. immunocompromised host) should influence treatment decisions.
Table 1. Bacterial Cause - Treatment Recommended
| Pathogen | Therapy Options |
|
Salmonella * GI Panel is unable to identify Salmonella species. With non-typhoidal streains, treatment may prolong shedding. * * Call micro lab for susceptibility testing as clinically indicated. |
Preferred: Ceftriaxone x 7 days Alternatives
|
| Shigella/Enteroinvasive E. coli (EIEC) |
Preferred: Azithromycin x 3 days Alternatives
|
Table 2. Bacterial Cause - Treatment Considered in Special Circumstances
| Pathogen | Treatment Considerations | Therapy Options |
| Campylobacter (jejuni, coli, and upsaliensis) |
Majority of infections are self-limiting and do not require antibiotic therapy Treatment recommended for special populations or severe disease:
|
Antibiotic options for special populations or severe disease: Preferred: Azithromycin x 3 days Alternative: Fluoroquinolone x 3 days (emergence of FQ-resistant Campylobacter after approval of these agents in poultry) |
| Plesiomonas shigelloides |
Majority of infections are self-limiting and do not require antibiotic therapy Treatment recommended for special populations or severe disease:
|
Antibiotic options for special populations or severe disease: Preferred: Azithromycin x 3 days Alternatives:
|
| Yersinia enterocolitica |
Majority of infections are self-limiting and do not require antibiotic therapy Treatment recommended for special populations or severe disease:
|
Antibiotic options for special populations or severe disease: Preferred: TMP/SMX x 3 days Alternatives:
|
| Vibrio (parahaemolyticus, vulnificus) |
Antibiotics not indicated in mild cases. No significant decrease in severity of illness or duration of diarrhea. Treatment recommended for persistent diarrhea (> 5 days) or invasive disease. Vibrio vulnificus may cause bacteremia / SSTI and treatment with empiric doxycycline is warranted in this setting. |
Antibiotic options for persistent diarrhea: Preferred: Doxycycline x 3 days Alternatives:
Invasive disease: Doxycycline 100 mg PO BID + Ceftriaxone 2 g IV daily x 7 days |
| Vibrio cholerae |
Antibiotics may be indicated in moderate to severe dehydration. Therapy reduces diarrhea ~50%, shortens duration of illness, and reduces risk of transmission. |
Antibiotic options in moderate to severe dehydration: Preferred: Doxycycline x 3 days Altnerative: Azithromycin x 3 days |
| Enterotoxigenic E. coli (ETEC) lt/st |
Antibiotics shown to shorten duration of illness, indicated for moderate to severe diarrhea. Consider in >4 stools per day, fever, or bloody stools. |
Antibiotic options for sepcial populations:
|
Table 3. Bacterial Cause - Treatment NOT Recommended
| Pathogen | Treatment Considerations | Therapy Options |
|
Diarrheagenic E. coli / Shigella Enteroaggregative E. coli (EAEC) Enteropathogenic E. coli (EPEC) |
Limited data. Generally self-limiting. Antibiotics not indicated. | Supportive care only |
|
Shiga-like toxin-producing E. coli (STEC) stx1/stx2 E. coli 0157 is a subtype of STEC and speciation will be reported for epidemiology interest. Does not impact management. |
Antibiotics and antimotility agents should be AVOIDED. | Supportive care only |
Table 4. Viral Cause - NO Treatment Recommended
| Pathogen | Treatment Considerations | Therapy Options |
| Adenovirus F 40/41 | Supportive care recommended | No antimicrobial therapy indicated |
| Astrovirus | Supportive care recommended | No antimicrobial therapy indicated |
| Norovirus GI/GII | Supportive care recommended |
No antimicrobial therapy indicated Antimotility agents may be useful |
| Rotavirus A | Supportive care recommended | No antimicrobial therapy indicated |
| Sapovirus (I, II, IV, and V) | Supportive care recommended | No antimicrobial therapy indicated |
Table 5. Parasitic Cause
| Pathogen | Treatment Considerations | Therapy Options |
| Cryptosporidium |
Treatment recommended for special populations or severe disease
|
Antiparasitic in special populations or in severe disease Preferred: Nitazoxanide x 3 days |
| Cyclospora cayetanensis | Treatment recommended |
Preferred: TMP/SMX x 7 days Alternative: Ciprofloxacin x 7 days |
| Entamoeba histolytica |
If asymptomatic, luminal agent recommended alone. In symptomatic patients, antiparasitic followed by luminal agent recommended. Treatment recommended. |
Asymptomatic: Paromomycin x 7 days Symptomatic Preferred: Metronidazole x 7-10 days followed by paromomycin x 7 days Alternative: Tinidazole x 3 days followed by paromomycin x 7 days |
| Giardia lamblia |
Only treat symptomatic patients. Antiparasitic agents have been shown to reduce duration of symptoms. If asymptomatic, treatment recommended for special populations:
|
Preferred: Tinidazole x 1 dose Alternative: Metronidazole x 5 days |
Diagnosis-Specific Information
Medication dosing recommendations
Doses listed below are all in the setting of normal renal function. For renal dosing adjustments, please refer to individual CustomID pages.
If specific dosing for indication differs from this chart, it will be outlined in the applicable Table.
| Medication | Dosing for standard renal function |
| Azithromycin | 500 mg PO daily |
| Ceftriaxone | 2g IV daily |
| Ciprofloxacin | 500 - 750 mg PO twice daily |
| Doxycycline | 100 mg PO twice daily |
| Levofloxacin | 750 mg PO daily |
| Metronidazole | 500 mg PO three times daily |
| Nitazoxanide | 500 mg PO twice daily |
| Paromomycin | 25-35 mg/kg PO divided into three doses daily |
| Tinidazole | 2 g PO daily |
| Trimethoprim-sulfamethoxazole (TMP-SMX) | 1 DS tablet PO twice daily |
References
- Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin Infect Dis. 2017;65(12):e45-e80. doi:10.1093/cid/cix669
- Bennet JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett’s Principles and Practices of Infectious Diseases. Chapter 98: Diarrhea With Little or no Fever. Chapter 99: Acute Dysentery Syndromes (Diarrhea with Fever). Chapter 100: Typhoid Fever, Paratyphoid Fever, and Typhoidal Fevers. Chapter 101: Foodborne Disease. (2019)
- The Sanford Guide to Antimicrobial Therapy. Sperryville, VA: Antimicrobial Therapy, Inc., 2021
- DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370(16):1532-1540. doi:10.1056/NEJMra1301069
- Connor B. Traveler’s Diarrhea. CDC Yellow Book 2024.